-
BridgeBio Announces Positive Phase 2 Cohort 5 Results of Infigratinib in Achondroplasia Demonstrating Mean Increase in Annualized Height Velocity of 3.03 cm/year with No Treatment-related Adverse Events
Source: Nasdaq GlobeNewswire / 06 Mar 2023 07:00:00 America/New_York
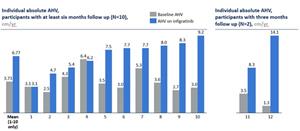
- In the highest dose level (Cohort 5, 0.25 mg/kg once daily), the mean change from baseline in annualized height velocity (AHV) at six months was +3.03 cm/yr (p = 0.0022) for the first 10 children with at least six months of follow-up in Cohort 5. The two remaining children who have not yet had six months of follow-up have a mean change from baseline in AHV of +8.8 cm/yr based on three months data
- 80% of children at six months were responders, as defined by an increase from baseline AHV of at least 25%. The mean change from baseline in AHV of responders was 3.81 cm/yr
- As a result of treatment, the median absolute AHV reached 7.6 cm/yr, which is beyond the 99th percentile of growth for children living with achondroplasia
- Infigratinib demonstrated clear dose-responsiveness as a single daily oral therapy and was well-tolerated with no adverse events (AEs) assessed as treatment-related in Cohort 5
- Based on the positive Phase 2 results, BridgeBio has started to enroll children for a pivotal Phase 3 trial
- BridgeBio expects to initiate clinical development of infigratinib for hypochondroplasia, a skeletal dysplasia closely related to achondroplasia and driven by fibroblast growth factor receptor 3 (FGFR3) gain-of-function variants, and will continue to explore the impact on the medical and functional complications of achondroplasia in future studies of infigratinib
PALO ALTO, Calif., March 06, 2023 (GLOBE NEWSWIRE) -- BridgeBio Pharma, Inc. (Nasdaq: BBIO) (BridgeBio), a commercial-stage biopharmaceutical company focused on genetic diseases and cancers, today announced positive results from PROPEL2, a Phase 2 trial of the investigational therapy infigratinib in children with achondroplasia, demonstrating potential best-in-class efficacy and a clean safety profile. Infigratinib is an oral small molecule designed to inhibit FGFR3 and target achondroplasia at its source. BridgeBio will also host an investor call on March 6, 2023, at 7:30 am ET to discuss the results from the Phase 2 study.
To date, key results from the clinical trial include:
- At the highest dose level evaluated to date (Cohort 5, 0.25 mg/kg once daily), the mean increase from baseline in annualized height velocity (AHV) for the 10 children that have had six-month visits was +3.03 cm/yr (p = 0.0022). Individual data can be found in Figure 1 below
- The baseline AHV for the 10 children with six-month visits was in the expected range for children with achondroplasia at 3.73 cm/yr, rising to 6.77 cm/yr after treatment
- The two remaining children who have not yet had six months of follow-up have a mean change from baseline in AHV of +8.8 cm/yr at three months. The mean age for the cohort was 7.24 years
- 80% of the 10 children with six-month visits were responders, with a change from baseline AHV of at least 25%. Among the responders, the average change from baseline in AHV was +3.81 cm/yr
- Preliminary analysis of Collagen X (CXM) levels also saw a statistically significant increase from baseline in Cohort 5 (p=.03). CXM is the gold-standard biomarker of chondrocyte-driven growth and further validates the robust response to infigratinib
- Combined with the previously reported Cohort 4 change from baseline in AHV value of +1.52 cm/yr, the Cohort 5 data demonstrate a strong dose response for infigratinib
- Median follow-up across all cohorts is 71.1 weeks. To date, the study has shown a well-tolerated safety profile, with no study drug related treatment emergent adverse events (TEAEs) in Cohort 5. No serious adverse events (SAEs) or discontinuations due to AEs were reported in any cohort
Figure 1
“The data from Cohort 5 has shown a major impact on annualized height velocity for children with achondroplasia and an excellent safety profile to date. We are thrilled to see these promising results and consider that AHV increases of this magnitude will translate to improvements in the medical and functional complications of achondroplasia. We are excited about taking the next steps towards initiating a Phase 3, pivotal clinical trial,” said Professor Ravi Savarirayan, M.D., Ph.D., clinical geneticist and group leader of molecular therapies research at the Murdoch Children’s Research Institute in Australia, the lead investigator for PROPEL2.
“I am encouraged by these efficacy and safety results and thankful for our partnership with the physicians, community advocates, children, and families in this study. These results reach a new tier of efficacy, and coupled with our differentiated safety and convenience profile, provide us the opportunity to serve children with achondroplasia and other skeletal dysplasias. We look forward to exploring the potential of infigratinib on the wider medical and functional impacts of achondroplasia, hypochondroplasia and other skeletal dysplasias, which hold significant unmet needs for families,” said Neil Kumar, Ph.D., founder and CEO of BridgeBio.
Based on the positive results to date, BridgeBio has started enrolling children in the run-in for a Phase 3 trial. Additionally, BridgeBio expects to initiate clinical development for infigratinib in hypochondroplasia, a skeletal dysplasia closely related to achondroplasia and similarly driven by FGFR3 gain-of-function variants. BridgeBio has previously presented promising preclinical data for hypochondroplasia at ENDO 2022 and ASHG 2022.
“Achondroplasia can have broad impact that affects the whole person. People can experience a range of medical complications, including foramen magnum stenosis, spinal stenosis, cardiovascular complications, sleep-disordered breathing, obesity, and sometimes, individuals may need surgical intervention. In addition to the potential medical and physical complications, people with achondroplasia may also experience social and emotional impacts as a result of living with the condition. We are encouraged by BridgeBio’s mission to develop a therapy with the potential to address this as a whole-person condition that affects the overall health, independent function, and quality of life of those with achondroplasia,” said Dianne Kremidas, executive director of The MAGIC Foundation.
Infigratinib has IP protection out to at least 2041.
Webcast Information
BridgeBio will host an investor call and simultaneous webcast to discuss the Phase 2 data from Cohort 5 of infigratinib in children with achondroplasia on March 6, 2023 at 7:30 am ET. A link to the webcast may be accessed from the event calendar page of BridgeBio’s website at https://investor.bridgebio.com/. A replay of the conference call and webcast will be archived on the Company’s website and will be available for at least 30 days following the event.About Achondroplasia
Achondroplasia is the most common cause of disproportionate short stature, affecting approximately 55,000 people in the United States (US) and European Union (EU), including up to 10,000 children and adolescents with open growth plates. Achondroplasia impacts overall health and quality of life, leading to medical complications such as obstructive sleep apnea, middle ear dysfunction, kyphosis, and spinal stenosis. The condition is uniformly caused by an activating mutation in FGFR3.About BridgeBio Pharma, Inc.
BridgeBio Pharma (BridgeBio) is a commercial-stage biopharmaceutical company founded to discover, create, test and deliver transformative medicines to treat patients who suffer from genetic diseases and cancers with clear genetic drivers. BridgeBio’s pipeline of development programs ranges from early science to advanced clinical trials. BridgeBio was founded in 2015 and its team of experienced drug discoverers, developers and innovators are committed to applying advances in genetic medicine to help patients as quickly as possible. For more information visit bridgebio.com and follow us on LinkedIn and Twitter.BridgeBio Pharma, Inc. Forward-Looking Statements
This press release contains forward-looking statements. Statements in this press release may include statements that are not historical facts and are considered forward-looking within the meaning of Section 27A of the Securities Act of 1933, as amended (the Securities Act), and Section 21E of the Securities Exchange Act of 1934, as amended (the Exchange Act), which are usually identified by the use of words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “projects,” “seeks,” “should,” “will,” and variations of such words or similar expressions. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act. These forward-looking statements, including statements relating to the clinical, therapeutic and market potential of our programs and product candidates, including our clinical development program for infigratinib in achondroplasia, the timing and success of our clinical development programs, the progress of our ongoing and planned clinical trials of infigratinib in achondroplasia and in hypochondroplasia, including our plans to initiate a Phase 3 trial for infigratinib in achondroplasia and to initiate clinical development in hypochondroplasia, our planned interactions with regulatory authorities, the availability of data from our clinical trials of infigratinib, and the timing of these events, reflect our current views about our plans, intentions, expectations and strategies, which are based on the information currently available to us and on assumptions we have made. Although we believe that our plans, intentions, expectations and strategies as reflected in or suggested by those forward-looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual results may differ materially from those described in the forward-looking statements and will be affected by a number of risks, uncertainties and assumptions, including, but not limited to, initial and ongoing data from our clinical trials not being indicative of final data, the design and success of ongoing and planned clinical trials, difficulties with enrollment in our clinical trials, adverse events that may be encountered in our clinical trials, the FDA or other regulatory agencies not agreeing with our regulatory approval strategies, components of our filings, such as clinical trial designs, conduct and methodologies, or the sufficiency of data submitted, potential adverse impacts due to the global COVID-19 pandemic such as delays in regulatory review, manufacturing and supply chain interruptions, adverse effects on healthcare systems and disruption of the global economy, the impacts of current macroeconomic and geopolitical events, including changing conditions from the COVID-19 pandemic, hostilities in Ukraine, increasing rates of inflation and rising interest rates, on our overall business operations and expectations, as well as those risks set forth in the Risk Factors section of our Annual Report on Form 10-K for the year ended December 31, 2022 and our other filings with the U.S. Securities and Exchange Commission. Moreover, we operate in a very competitive and rapidly changing environment in which new risks emerge from time to time. These forward-looking statements are based upon the current expectations and beliefs of our management as of the date of this press release, and are subject to certain risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements. Except as required by applicable law, we assume no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise.BridgeBio Contact:
Vikram Bali
contact@bridgebio.com
(650)-789-8220BridgeBio Skeletal Dysplasia Advocacy Contact:
Anne Lee
Anne.Lee@bridgebio.com
(415)-694-0979A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/a43bfd09-35ca-4f7a-925f-4e5b114b7837